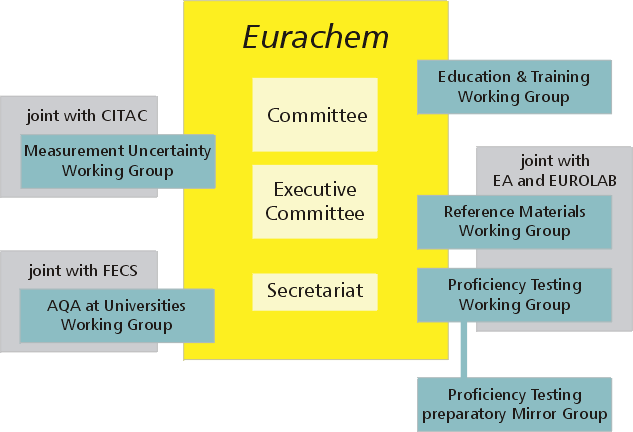
About twenty years after the first symposium "Production
and Use of Reference Materials" at the Federal
Institute for Materials Research and Testing (BAM) in
November 1979, an international symposium "Reference
Materials for Technologies in the New Millennium"
took place again in Berlin on 22 - 23 May 2000. This year's
symposium which was arranged by EUROLAB Germany together
with BAM was again intended to provide an opportunity for
the international exchange of information on, and
experience with reference materials (RM) between RM
producers and users. The nearly 150 symposium
participants came from 27 European, American and Asian
countries, among them experts from Mongolia, China,
Brazil, Mexico, and the United States.
Challenging Programme
The aim of the symposium was to bring the interested
parties together and to foster development of a concerted
approach to the development, distribution, and use of
reference materials. Due to the broad response to the
call for papers, the Organizing Committee received not
only papers on RM for materials analysis but also
contributions related to a variety of other kinds of RM
and to more general questions concerning the development,
certification, and application of RM. Therefore, the
contributions (28 lectures and 32 posters) covered the
topics:
* General Aspects
* Pure Materials and Elemental Solutions
* Inorganic Matrix RM
* RM for Physico-Chemical Properties
* RM for Physical Properties
* Environmental RM
* Organic, Biological, and Clinical RM.
The symposium programme comprised six sessions arranged
according to the above mentioned topics and one poster
session. After the Welcoming address by the
representative of the German Federal Ministry of
Economics and Technology, Staatssekretär A. Tacke, the
opening lecture was given by Horst Czichos, President of
EUROLAB and President of BAM. He presented the current
activities of BAM in the field of RM.
The first session (Chair: A. Zschunke, BAM) was opened by
Reenie Parris from the NIST Analytical Chemistry Division
with a survey on NIST SRM, NTRM and SRD as tools for
facilitating SI-traceable chemical measurements in the
New Millennium. The other lectures in the first session
concerned the establishment of traceability in chemical
measurements and RM (P. de Bievre, IRMM, Belgium) as well
strategies for developing reference materials (Y. Mitani,
Centro Nacional de Metrología, Mexico).

The opening lecture on RM for 21st century
technologies was given by BAM and EUROLAB president Horst
Czichos.

Lively discussions in the plenary sessions.
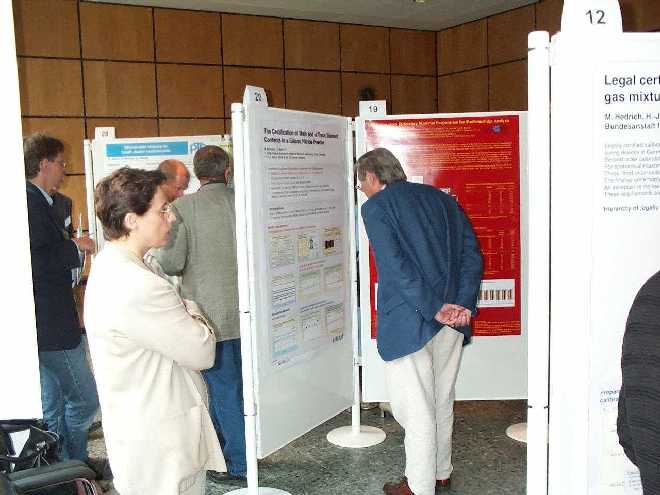
During the well-attended poster session.
Uncertainty and Traceability Issues
In the second session (Chair: M. Grasserbauer, IRMM)
lectures on RM as transfer standard in the traceability
chain (H. Felber, EMPA, Switzerland), on questions of the
uncertainty of RM according to the requirements of the
"Guide to the expression of uncertainty in
measurement" (J. Pauwels, IRMM) and on different
ways to declare the quality of RM (M. Golze, BAM) were
given.
The third session (Chair: R. Worswick, LGC, U.K.) was
dominated by contributions on RM for elemental analysis,
such as on the BAM system of primary calibration
substances for establishing traceability in the field of
element analysis (R. Matschat, BAM), on the certification
of pure materials and elemental solutions (R. Lawn, LGC)
and on purified metals as a tool to achieve traceability
to the S.I. (G. Fortunato, EMPA). Finally, P. D. P.
Taylor (IRMM) presented a contribution on isotopic spike
calibration solutions for isotope dilution.
There was an opportunity for a short discussion after all
of the lectures in each session which was well used by
the participants. The first day of the scientific
programme was finished with the poster session. It was
well attended and also a good place for interesting
discussions.
New Concepts, New Materials
The lectures on the second day of the symposium concerned
a variety of new reference materials for very different
purposes such as industrial, environmental, medical, and
biological RM (session IV, Chair: M. Walsh, State
Laboratory, Ireland) as well RM for physical, mechanical,
and physico-chemical properties (session V, Chair: N.
Trahey , NIST together with H. Klich, BAM, and session VI,
Chair: E. de Leer, NMi).
H. Muntau from JRC in Ispra (Italy) introduced new
concepts for laboratory reference materials (LRM) and
their role in environmental analysis. Low cost RM for
routine use in food testing labs were presented by P.
Roper (LGC). Further lectures of the fourth session
reported about the Russian system of state RM for water
quality control (A. N. Atanov, WRCC, St. Petersburg),
about biological RM for air monitoring (K. Hoppstock, FZ
Jülich, Germany), about EC RM for in-vitro diagnostics
needs generated by the IVD-MD directive (H. Schimmel,
IRMM), and about nuclear environmental RM (A. Held, IRMM,
presented by P. D. P. Taylor).
The certification of porous RM was the topic of K. Meyer
(BAM) in session V, whereas the lectures of J. Kelly (NIST)
and W. Hinrichs (MPA Clausthal, Germany) dealt with
various problems of RM for particle size distribution. C.
du Fresne von Hohenesche (University of Mainz, Germany)
reported about a HPLC column as a RM, C. Ingelbrecht
compared EC RM for impact toughness with other RM
available in the world.
At the end of this extensive session some problems of the
certification of ion-implanted shallow layers in Si-wafers
were pointed out by R. Klockenkämper (ISAS, Germany).
Finally, in the last session of the symposium RM for pH
and electrolytic conductivity (P. Spitzer, PTB, Gemany),
EURONORM certified RM for environmentally sensitive
elements in steels (R. P. Meeres, BAS, U.K.), as well as
gaseous primary RM and its IDMS analysis (E.W.B. de Leer,
NMi) were presented.
Successful Event
A summary and closing remarks from the point of view of a
participant to the symposium were given by J. Pauwels (IRMM).
He reflected on the situation in the field of RM in 1979
when the first RM symposium took place at BAM and on the
remarkable progress since that time which was
demonstrated at this year's symposium.
J. Pauwels elaborated: "Twenty years ago when we
were talking about reference materials, we were
especially talking about materials which were useful to
develop the industrial process: steel industry, non-ferrous
metals industry, nuclear industry.
Today we are coming closer and closer to reference
materials which concern everybody of the public, which
concern our life, which concern our health. Meanwhile, we
have learned making reference materials for complex
matrices, and we have also discovered other important
things: that there is something like instability and we
have to monitor stability, that we have to look at
homogeneity, that the increasing demands in analytical
chemistry require us to give more information relevant
for, and important in quality assurance and quality
control.
We have explored traceability to whatever standards are
available. We have learned that uncertainty is something
more than a standard deviation. Very long time ago we
were happy to quote repeatability or maybe
reproduceability as uncertainty. Today we know that
uncertainty means something else and with the increasing
requirements to analytical chemistry all of us have
realised that also we as producers of reference materials
have to make efforts to give more complete information.
There are lots of things which came upon us, things like
accreditation, now we should try to get accreditation
systems recognised just as our measurements have to be
recognised, and just as our reference materials have to
be recognised."
With many thanks to the organizers, J. Pauwels closed
this successful symposium which was accompanied - besides
the scientific programme - by an exhibition of
commercially available RM and an excellent symposium
dinner on a sightseeing boat on the river Spree.
Peter Klobes
Federal Institute for
Materials Research and Testing (BAM)
Parties interested in more details may order the book
of abstracts directly from:
EUROLAB Germany, Secretariat
Unter den Eichen 87, D-12205 Berlin, Germany.
|