The Fitness for Intended Use of Analytical Equipment and Systems (1st Ed)
The Fitness for Intended Use of Analytical Equipment and Systems - A Laboratory Guide to the Life Cycle of Analytical Equipment and Systems, their Qualification and Related Topics
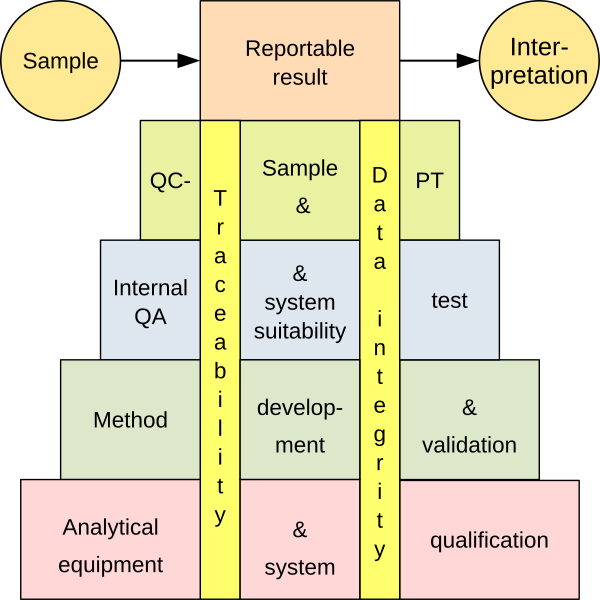 A wide variety of analytical equipment is used in analytical laboratories, ranging from simple apparatus to complex computer-based systems, to collect data that helps to obtain a reportable result. Many of these pieces of equipment combine a measurement function with software control. There are many ways to demonstrate that an equipment is qualified and under control, including qualification, calibration, validation and maintenance. To ensure ‘fitness for purpose’, an integrated approach based on risk assessment is recommended.
A wide variety of analytical equipment is used in analytical laboratories, ranging from simple apparatus to complex computer-based systems, to collect data that helps to obtain a reportable result. Many of these pieces of equipment combine a measurement function with software control. There are many ways to demonstrate that an equipment is qualified and under control, including qualification, calibration, validation and maintenance. To ensure ‘fitness for purpose’, an integrated approach based on risk assessment is recommended.
For the purposes of this guide, the term ‘equipment’ includes all apparatus, devices, instruments or instrument systems used in chemical, physical or biological analyses. The instruments form the basis for analytical work, from the development of the method to its validation and routine use. Without this stable foundation, the reliability of the entire process is questionable.
This document is a laboratory guide to the life cycle of analytical equipment and systems and their qualification for laboratories working to various standards and regulations. It is designed to help the laboratory management and staff and other users of analytical equipment to find a common nomenclature to meet the requirements efficiently, to do their work efficiently and to better understand the characteristics and limitations of the equipment.
The guide covers the entire lifespan of the equipment. It begins with the design, development and production of new equipment by the manufacturer. At the user's end, it covers all processes from the considerations involved in purchasing new equipment to commissioning, operation, maintenance, requalification and decommissioning. The main focus of attention is on the fitness for the intended use of the analytical equipment.
Availability
This guidance is available in the following languages:
- Download the guide in English (published 2025-12-18) (pdf, 1.8 Mb).
Citation
This publication should be cited* as:
Ernst P. Halder (ed.) Eurachem Guide: The Fitness for Intended Use of Analytical Equipment and Systems – A Laboratory Guide to the Life Cycle of Analytical Equipment and Systems, their Qualification and Related Topics (1st ed. 2025). ISBN 978-0-948926-41-9. Available from http://www.eurachem.org
*Subject to journal requirements
Translations
Translation into other languages is permitted for members of Eurachem. Other offers of translation should be directed to the Eurachem Secretariat for permission. The Eurachem policy on maintenance and development of Eurachem guidance, available on the Policies page, gives further information on translation.
Feedback
The working group is very interested in feedback from readers, particularly from those who are directly involved in using equipment. Comments can be provided via the AE&SQ Contact Form on this website.
- Details
- Last Updated: Friday, 19 December 2025 22:16
