- Details
-
Published: Saturday, 10 July 2010 22:05
Contents
This guide has been produced by a joint EURACHEM/CITAC Measurement Uncertainty Working Group.
 The first edition of the EURACHEM Guide for “Quantifying Uncertainty in Analytical Measurement” was published in 1995 based on the ISO "Guide to the Expression of Uncertainty in Measurement". The second edition was prepared in collaboration with CITAC in 2000 in the light of practical experience of uncertainty estimation in chemistry laboratories and the even greater awareness of the need to introduce formal quality assurance procedures by laboratories. The second edition stressed that the procedures introduced by a laboratory to estimate its measurement uncertainty should be integrated with existing quality assurance measures, since these measures frequently provide much of the information required to evaluate the measurement uncertainty.
The first edition of the EURACHEM Guide for “Quantifying Uncertainty in Analytical Measurement” was published in 1995 based on the ISO "Guide to the Expression of Uncertainty in Measurement". The second edition was prepared in collaboration with CITAC in 2000 in the light of practical experience of uncertainty estimation in chemistry laboratories and the even greater awareness of the need to introduce formal quality assurance procedures by laboratories. The second edition stressed that the procedures introduced by a laboratory to estimate its measurement uncertainty should be integrated with existing quality assurance measures, since these measures frequently provide much of the information required to evaluate the measurement uncertainty.
This third edition retains the features of the second edition and adds information based on developments in uncertainty estimation and use since 2000. The additional material provides:
- Improved guidance on the expression of uncertainty near zero;
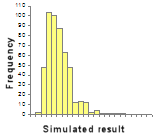
- New guidance on the use of Monte Carlo methods for uncertainty evaluation;
- Improved guidance on the use of proficiency testing data:
- Improved guidance on the assessment of compliance of results with measurement uncertainty.
The guide therefore provides explicitly for the use of validation and related data in the construction of uncertainty estimates in full compliance with the formal ISO Guide principles set out in the ISO Guide to the Expression of Uncertainty in measurement. The approach is also consistent with the requirements of ISO/IEC 17025:2005.
Availability
You may download the guide from this website at no cost (pdf, 1.908 MB).
NB: See also "Comments and errata" below.
Citation
This guidance should be cited* as
"S L R Ellison and A Williams (Eds). Eurachem/CITAC guide: Quantifying Uncertainty in Analytical Measurement, Third edition, (2012) ISBN 978-0-948926-30-3. Available from www.eurachem.org."
*Subject to journal requirements.
Translations
Available translations
The current (third) edition of this guide is currently available for download in English Farsi*, Spanish* and Japanese*.
Note 1. Translation first published 2018-08-06: editorial amendment published 2022-07-31
In addition:
See also "Previous editions", below, for earlier translations.
Translating this guide
Eurachem members are entitled to prepare translations of Eurachem guidance in their own language. Other organisations or individuals must request permission from the Eurachem Executive to undertake or publish translations. The Eurachem policy on maintenance and development of Eurachem guidance, available on the Policies page, gives further information on translation.
Comments and errata
Since publication of the third edition of the Guide in 2012, a number of minor typographical issues have been identified. Although not sufficient to justify revision and re-issue of the complete Guide and translations, some affect the detailed results of calculations in examples, making it hard to check results exactly. The Measurement Uncertainty and Traceability WG have accordingly prepared a list of comments and corrections for reader information. These are likely to be implemented in future editions.
Previous editions
The previous editions in English are available from this website:
Some translations of earlier editions are also available from respective national members or elsewhere. The 2nd edition has been translated into Italian, Portuguese, Russian and Swedish. The Italian translation of the 2nd edition can be downloaded from the Eurachem Guide page on the INRIM website. For details about the translation of the 2nd edition of the Guide into Russian please contact This email address is being protected from spambots. You need JavaScript enabled to view it. from the D. I. Mendeleyev Institute for Metrology. The 1st edition (1995) has been translated into Czech, Lithuanian, Russian and Spanish. For details about older translations please contact the Eurachem representative in the respective country or the Eurachem Secretariat
Acknowledgements
Production of the English edition of the guide was supported by the UK National Measurement System.
The Spanish translation of the 3rd edition was provided by P Morillas (Spain) and the Farsi translation by Mohammad Rahmani (Iran).
The Japanese translation is also commercially available from the publisher Maruzen Publishing: see https://www.maruzen-publishing.co.jp/ or Amazon Japan.
Contact point
Any comment or query about the content of this guide should be directed to the Working Group Secretary.
Figures
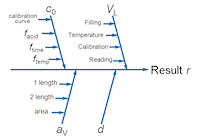
1. Cause and Effect diagram: Uncertainty sources in leachable cadmium measurement
2. A histogram of 500 Monte Carlo simulations. See Appendix E.3 in the Guide for details


 Measurement uncertainty arising from sampling: A guide to methods and approaches
Measurement uncertainty arising from sampling: A guide to methods and approaches The first edition of the EURACHEM Guide for “Quantifying Uncertainty in Analytical Measurement” was published in 1995 based on the ISO "Guide to the Expression of Uncertainty in Measurement". The second edition was prepared in collaboration with CITAC in 2000 in the light of practical experience of uncertainty estimation in chemistry laboratories and the even greater awareness of the need to introduce formal quality assurance procedures by laboratories. The second edition stressed that the procedures introduced by a laboratory to estimate its measurement uncertainty should be integrated with existing quality assurance measures, since these measures frequently provide much of the information required to evaluate the measurement uncertainty.
The first edition of the EURACHEM Guide for “Quantifying Uncertainty in Analytical Measurement” was published in 1995 based on the ISO "Guide to the Expression of Uncertainty in Measurement". The second edition was prepared in collaboration with CITAC in 2000 in the light of practical experience of uncertainty estimation in chemistry laboratories and the even greater awareness of the need to introduce formal quality assurance procedures by laboratories. The second edition stressed that the procedures introduced by a laboratory to estimate its measurement uncertainty should be integrated with existing quality assurance measures, since these measures frequently provide much of the information required to evaluate the measurement uncertainty.
 This document represents the current state-of-the-art with respect to the selection and use of proficiency testing schemes, and the interpretation of results and evaluations given in proficiency testing schemes. Although aimed primarily at staff in analytical laboratories, it is also useful for customers of laboratories, assessors working for accreditation bodies and other external users of PT scheme results.
This document represents the current state-of-the-art with respect to the selection and use of proficiency testing schemes, and the interpretation of results and evaluations given in proficiency testing schemes. Although aimed primarily at staff in analytical laboratories, it is also useful for customers of laboratories, assessors working for accreditation bodies and other external users of PT scheme results.
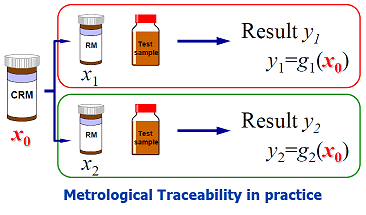 This Guide gives detailed guidance for the establishment of metrological traceability in quantitative chemical analysis, based on the definition in the international vocabulary of basic and general terms in metrology (VIM). Though it is aimed principally at testing and measurement laboratories carrying out chemical measurement, the principles are expected to apply from routine analysis to basic research. The document is intended to assist laboratories in meeting the requirements on traceability of results given in ISO/IEC 17025.
This Guide gives detailed guidance for the establishment of metrological traceability in quantitative chemical analysis, based on the definition in the international vocabulary of basic and general terms in metrology (VIM). Though it is aimed principally at testing and measurement laboratories carrying out chemical measurement, the principles are expected to apply from routine analysis to basic research. The document is intended to assist laboratories in meeting the requirements on traceability of results given in ISO/IEC 17025.
 This guide has been produced by members of the Eurachem Education and Training Working Group and others co-opted to the Project group for this task.
This guide has been produced by members of the Eurachem Education and Training Working Group and others co-opted to the Project group for this task.